Is Topical Dissolver the Next Big Thing in Plastic Surgery?
A closer look at Topilase-- the hyaluronidase cream being marketed for unwanted filler in the under eye area
With the rise of plastic surgery and the ensuing rise of injectables, we have seen an ensuing a spike in filler complications: puffy eyes, bumps on lips, and generally dysmorphic features. To me, this represents a waning of aesthetic judgement, a failure to stay true to anatomy, and a misunderstanding about both the the benefits and the limitations of what injectable fillers can do.
Regardless, more filler writ-large means a greater need for dissolver. We currently use injectable hyaluronidase as the main tool in our toolbox for these situations— not just vascular occlusion or tissue threat, but also for undesirable aesthetic appearance.
And now in the US, we have Topilase. What does it mean, and will we use it?
What is Topilase?
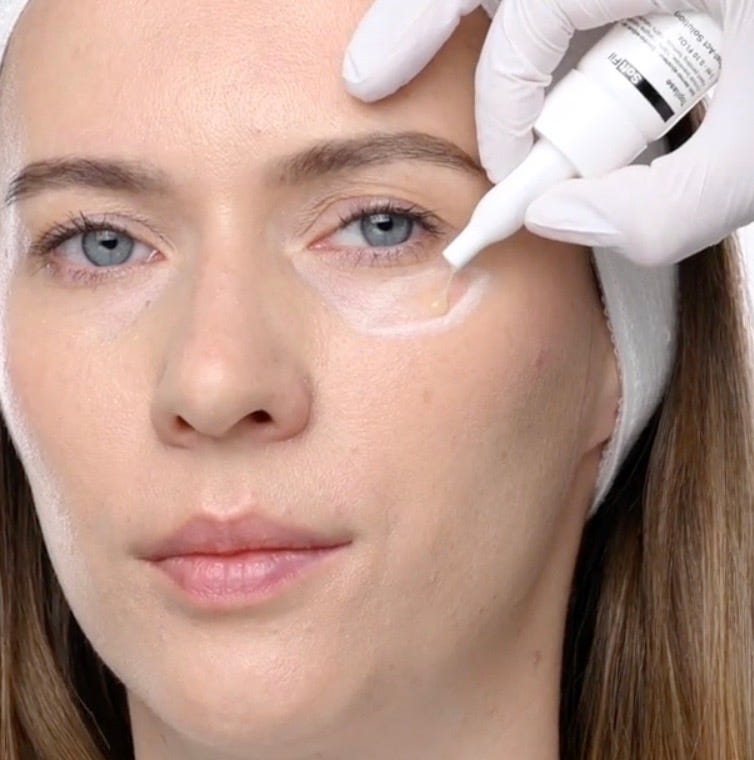
Topilase is a topical formulation that contains hyaluronidase, an enzyme that breaks down hyaluronic acid (HA). Hyaluronic acid is a naturally occurring substance in the skin, known for its ability to retain moisture and add volume. In aesthetic treatments, HA-based dermal fillers are commonly used to address wrinkles, fine lines, and volume loss. However, complications can arise, necessitating the removal or adjustment of these fillers. Topilase is marketed as a non-invasive solution to manage such issues by dissolving excess HA.
Mechanism of Action
Topilase works by utilizing hyaluronidase to target and degrade hyaluronic acid molecules. When applied to the skin, the enzyme penetrates the dermal layers and breaks down HA into smaller fragments, which are then naturally metabolized by the body. This process reduces the volume of HA-based fillers, allowing for precise adjustments or complete removal without the need for invasive procedures. The effectiveness of Topilase hinges on its ability to deliver the enzyme to the targeted area efficiently and safely.
Topilase contains not just hyaluronidase, but also protease (an enzyme that dissolves protein), and lipase (an enzyme that dissolves fat). The manufacturer packaging states that these enzymes have no effect on tissue, but this is perplexing. Why include these potentially problematic ingredients if they serve no purpose? The notion that native protein and fat may be dissolved is also a concern.
Interesting Features of Topilase
1. Non-Invasive Adjustment of Fillers:
Topilase offers a non-surgical method to modify or remove HA-based dermal fillers. This feature is particularly beneficial for patients who experience overfilling, asymmetry, or other complications from filler treatments. The topical application eliminates the need for injections, reducing discomfort and recovery time.
2. Less discomfort
As a topical cream, Topilase is more comfortable for patients that injected hyaluronidase.
3. Potential for Broader Applications:
While primarily designed for adjusting dermal fillers, the enzymatic action of Topilase could potentially be adapted for other dermatological applications including excessive HA accumulation, malar edema, fibrosis, and even for use in vascular occlusions where a zone of tissue needs to be treated.
Global Availability and Regulatory Status
Topilase is marketed as a cosmetic rather than a drug, which influences its packaging and the claims that can be made about its efficacy. As a cosmetic product, its packaging contains no explicit claims about its effects or uses, adhering to regulations that differ from those governing pharmaceutical products. Currently, Topilase is available in several countries, including parts of Europe and Asia. However, it has not yet received approval for use in the United States. The regulatory landscape and accessibility of Topilase may vary, impacting its availability to practitioners and patients.
Drawbacks and Concerns
1. Variable Efficacy:
The effectiveness of Topilase can vary depending on factors such as the depth and concentration of HA fillers, the formulation of the product, and individual patient characteristics. This variability can theoretically lead to inconsistent results, necessitating multiple applications.
2. Risk of Over-Correction:
As with any treatment involving enzymatic degradation, there is a risk of over-correction. If too much HA is broken down, it can result in volume loss or uneven texture, potentially requiring additional treatments to restore the desired appearance.
3. Risk of Effects on Native Tissue
It’s not clear from the limited available data if Topilase will harm human hyaluronic acid, fat, or protein.
4. Potential Side Effects:
While generally considered safe, adverse reactions, including allergic responses, skin irritation, or swelling are possible.
5. Lack of Precision:
One significant drawback of Topilase is the lack of precision due to its topical application. Unlike injections that can target specific areas, the massage application method can result in a broader and less controlled distribution of the enzyme, potentially affecting unintended areas.
6. Additional Enzymes:
A concerning aspect of Topilase is that it contains not only hyaluronidase but also lipase and protease. These additional enzymes can break down fats and proteins, respectively, raising concerns about unintended tissue degradation and potential side effects.
So where does this leave us?
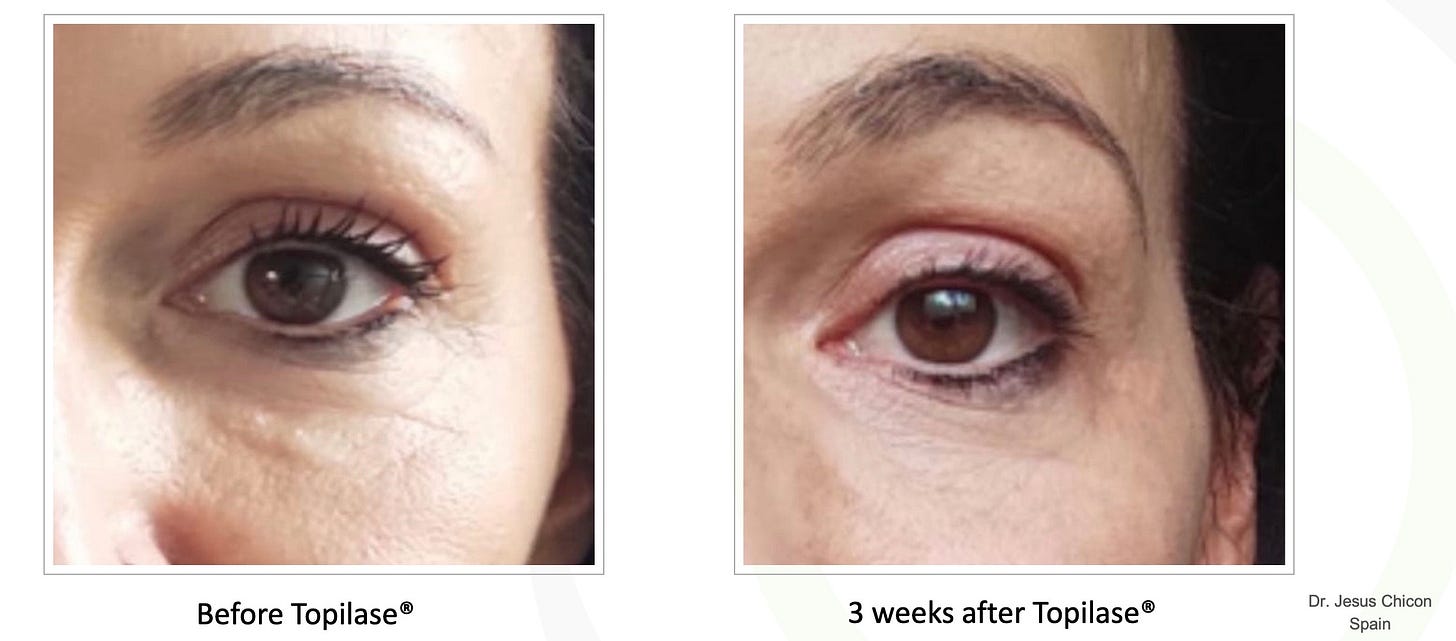
Topilase represents an advancement in the field of aesthetic medicine, offering a non-invasive solution for the adjustment and removal of HA-based dermal fillers. Its mechanism of action, centered around the enzymatic breakdown of hyaluronic acid, provides an imprecise and non-controlled mechanism for managing filler complications, however. While the product boasts numerous benefits, including convenience and lack of pain, it also presents challenges such as variable efficacy, risk of over-correction, and limited accessibility. Furthermore, the presence of additional enzymes like lipase and protease introduces potential risks that require careful consideration. As research and clinical experience with Topilase continue to evolve, it holds possible promise for improving the outcomes and safety of aesthetic treatments. As for me, I will plan to stock it in a filler emergency kit for potential use for vascular occlusion or extenuating circumstances— however I don’t plan to use it when injected hyaluronidase will serve, at this time.